Arrangement Of Electrons In Atoms
2.6 Arrangements of Electrons
Learning Objective
- Describe how electrons are grouped within atoms.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said fiddling about how electrons occupy the space about the nucleus. Practise they move around the nucleus at random, or do they be in some ordered arrangement?
The modern theory of electron behavior is called breakthrough mechanicsThe modern theory of electron behavior. . It makes the following statements well-nigh electrons in atoms:
- Electrons in atoms can take only certain specific energies. Nosotros say that the energies of the electrons are quantizedHaving a fixed value. .
- Electrons are organized according to their energies into sets called shellsA grouping of electrons within an atom. . Generally the college the free energy of a beat out, the farther information technology is (on boilerplate) from the nucleus. Shells practice not have specific, fixed distances from the nucleus, simply an electron in a college-free energy shell will spend more time further from the nucleus than does an electron in a lower-energy trounce.
- Shells are further divided into subsets of electrons called subshellsA group of electrons within a beat. . The showtime trounce has but one subshell, the 2nd vanquish has two subshells, the third vanquish has three subshells, and so on. The subshells of each beat are labeled, in order, with the letters southward, p, d, and f. Thus, the first vanquish has only an southward subshell, the second shell has an s and a p subshell, the third shell has s, p, and d subshells, and so forth.
- Dissimilar subshells hold a different maximum number of electrons. Any south subshell tin can hold upward to 2 electrons; p, 6; d, 10; and f, 14.
It is the arrangement of electrons into shells and subshells that about concerns us here, and then we will focus on that.
We use numbers to betoken which shell an electron is in. The start shell, closest to the nucleus and with the lowest-energy electrons, is beat out i. This first beat has only ane subshell, which is labeled s and can hold a maximum of ii electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, considering a hydrogen cantlet has its single electron in the s subshell of the first shell, nosotros employ 1s one to describe the electronic structure of hydrogen. This structure is chosen an electron configurationA shorthand description of the arrangement of electrons in an atom. . Electron configurations are shorthand descriptions of the arrangements of electrons in atoms. The electron configuration of a hydrogen cantlet is spoken out loud as "one-ess-one."
Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because southward subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as "ane-ess-ii").
The isouthward subshell cannot hold iii electrons (because an southward subshell tin can hold a maximum of 2 electrons), so the electron configuration for a lithium cantlet cannot be 1s three. Two of the lithium electrons tin fit into the 1s subshell, but the 3rd electron must become into the second shell. The 2d shell has two subshells, due south and p, which fill up with electrons in that gild. The 2s subshell holds a maximum of two electrons, and the twop subshell holds a maximum of 6 electrons. Because lithium's terminal electron goes into the twosouth subshell, we write the electron configuration of a lithium atom as 1due south 2iidue south 1.
The adjacent largest atom, beryllium, has iv electrons, so its electron configuration is ones 22south ii. Now that the 2s subshell is filled, electrons in larger atoms start filling the 2p subshell. Thus, the electron configurations for the side by side 6 atoms are as follows:
B: 1due south 22southward two2p 1
C: 1s ii2south 22p ii
North: 1southward 22s two2p three
O: 1south 22s 22p 4
F: 1south ii2due south 2twop 5
Ne: is ii2s ii2p 6
With neon, the iip subshell is completely filled. Because the second shell has only two subshells, atoms with more electrons at present must brainstorm the tertiary trounce. The third trounce has three subshells, labeled s, p, and d. The d subshell tin can concord a maximum of 10 electrons. The first two subshells of the third beat out are filled in order—for instance, the electron configuration of aluminum, with 13 electrons, is anes 22due south 22p 63s 23p 1. However, a curious thing happens after the threep subshell is filled: the fourdue south subshell begins to fill before the 3d subshell does. In fact, the verbal ordering of subshells becomes more complicated at this point (after argon, with its 18 electrons), so we will not consider the electron configurations of larger atoms.
A fourth subshell, the f subshell, is needed to consummate the electron configurations for all elements. An f subshell can agree up to 14 electrons.
Case 7
What is the electron configuration of a neutral phosphorus atom?
Solution
A neutral phosphorus cantlet has 15 electrons. Two electrons tin go into the 1s subshell, 2 tin can go into the 2due south subshell, and 6 can become into the 2p subshell. That leaves 5 electrons. Of those 5 electrons, two tin get into the 3s subshell, and the remaining iii electrons can go into the 3p subshell. Thus, the electron configuration of neutral phosphorus atoms is 1s 22southward 22p 6iiis 2threep iii.
Skill-Building Exercise
-
What is the electron configuration of a neutral chlorine atom?
Chemistry results from interactions between the outermost shells of electrons on different atoms. Thus, it is convenient to carve up electrons into two groups. Valence shell electronsAn electron in the highest-numbered shell of an atom. (or, more than simply, the valence electrons) are the electrons in the highest-numbered shell, or valence vanquishThe highest-numbered beat of an cantlet that contains electrons. , while cadre electronsAn electron in a lower-numbered shell of an atom. are the electrons in lower-numbered shells. We tin see from the electron configuration of a carbon atom—1s 2iis 2iip ii—that information technology has four valence electrons (iis 2iip 2) and ii cadre electrons (onedue south 2).
Case 8
From the electron configuration of neutral phosphorus atoms in Example vii, how many valence electrons and how many cadre electrons does a neutral phosphorus atom accept?
Solution
The highest-numbered shell is the third crush, which has ii electrons in the 3s subshell and 3 electrons in the 3p subshell. That gives a total of 5 electrons, and then neutral phosphorus atoms have five valence electrons. The 10 remaining electrons, from the first and second shells, are core electrons.
Skill-Building Practice
-
From the electron configuration of neutral chlorine atoms (see the Skill-Edifice Do following Example vii), how many valence electrons and how many core electrons does a neutral chlorine atom have?
Concept Review Exercises
-
How are electrons organized in atoms?
-
What data does an electron configuration convey?
-
What is the difference between core electrons and valence electrons?
Answers
-
Electrons are organized into shells and subshells around nuclei.
-
The electron configuration states the arrangement of electrons in shells and subshells.
-
Valence electrons are in the highest-numbered shell; all other electrons are core electrons.
Fundamental Takeaway
- Electrons are organized into shells and subshells nearly the nucleus of an atom.
Exercises
-
What is the maximum number of electrons that can fit in an s subshell? Does it affair what beat the s subshell is in?
-
What is the maximum number of electrons that can fit in a p subshell? Does it matter what shell the p subshell is in?
-
What is the maximum number of electrons that tin fit in a d subshell? Does it matter what shell the d subshell is in?
-
What is the maximum number of electrons that tin can fit in an f subshell? Does it matter what vanquish the f subshell is in?
-
What is the electron configuration of a carbon atom?
-
What is the electron configuration of a sulfur atom?
-
What is the valence beat electron configuration of a calcium atom?
-
What is the valence trounce electron configuration of a selenium atom?
-
What atom has the electron configuration 1s ii2s 22p 5?
-
What cantlet has the electron configuration anedue south 22s two2p 6threesouth ii3p three?
-
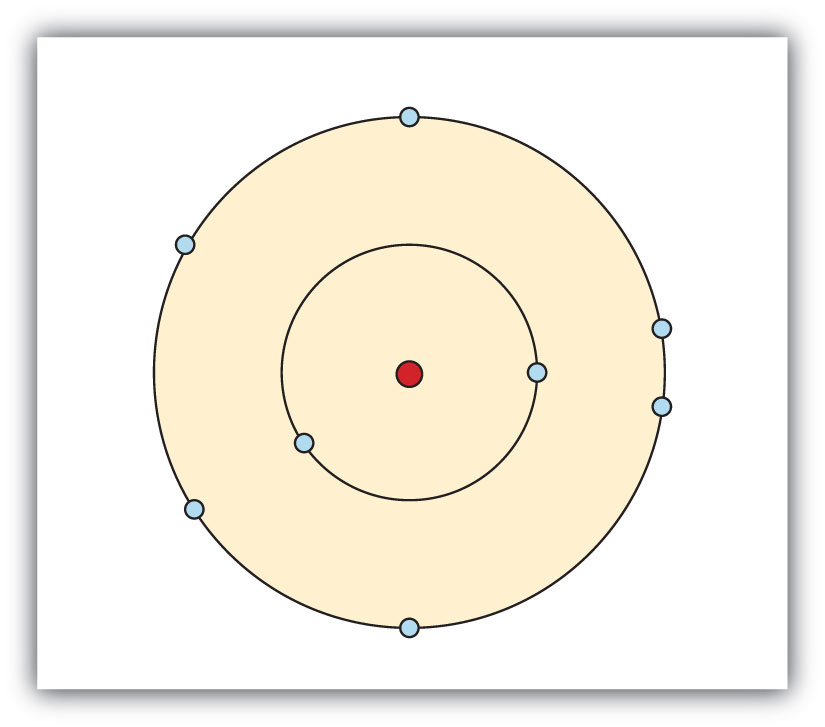
Depict a representation of the electronic structure of an oxygen atom.
-
Describe a representation of the electronic structure of a phosphorus atom.
-
A potassium atom has ____ cadre electrons and ____ valence electrons.
-
A silicon cantlet has ____ core electrons and ____ valence electrons.
Answers
-
2; no
-
10; no
-
1due south 2iidue south 2iip 2
-
ivsouth 2
-
fluorine
-
-
18; 1
Arrangement Of Electrons In Atoms,
Source: https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/s05-06-arrangements-of-electrons.html
Posted by: griffinthaddens.blogspot.com


0 Response to "Arrangement Of Electrons In Atoms"
Post a Comment